Synova WAVE
$100,000.00
Supercharge your fat processing
• 3-minute processing
• High viability
• Speed, quality, and simplicity
*Normal Delivery Time is 3 to 6 weeks after order. Certain orders may take up to 12 weeks for shipment to be made after order.
SkinStim TM Warnings and Precautions – Click Here
📌 What It Is
The Synova WAVE is a bedside adipose (fat) tissue processing system designed to extract a patient’s own regenerative cells — including stem cells, endothelial cells, and regenerative proteins — from fat tissue collected via a mini-liposuction procedure. It’s a closed, sterile device intended for point-of-care use in clinics and medical practices.
⚙️ Key Features & Technology
⏱️ Rapid Processing
-
Processes harvested fat in approximately 3 minutes, dramatically faster than traditional methods.
💪 High Cell Viability & Yield
-
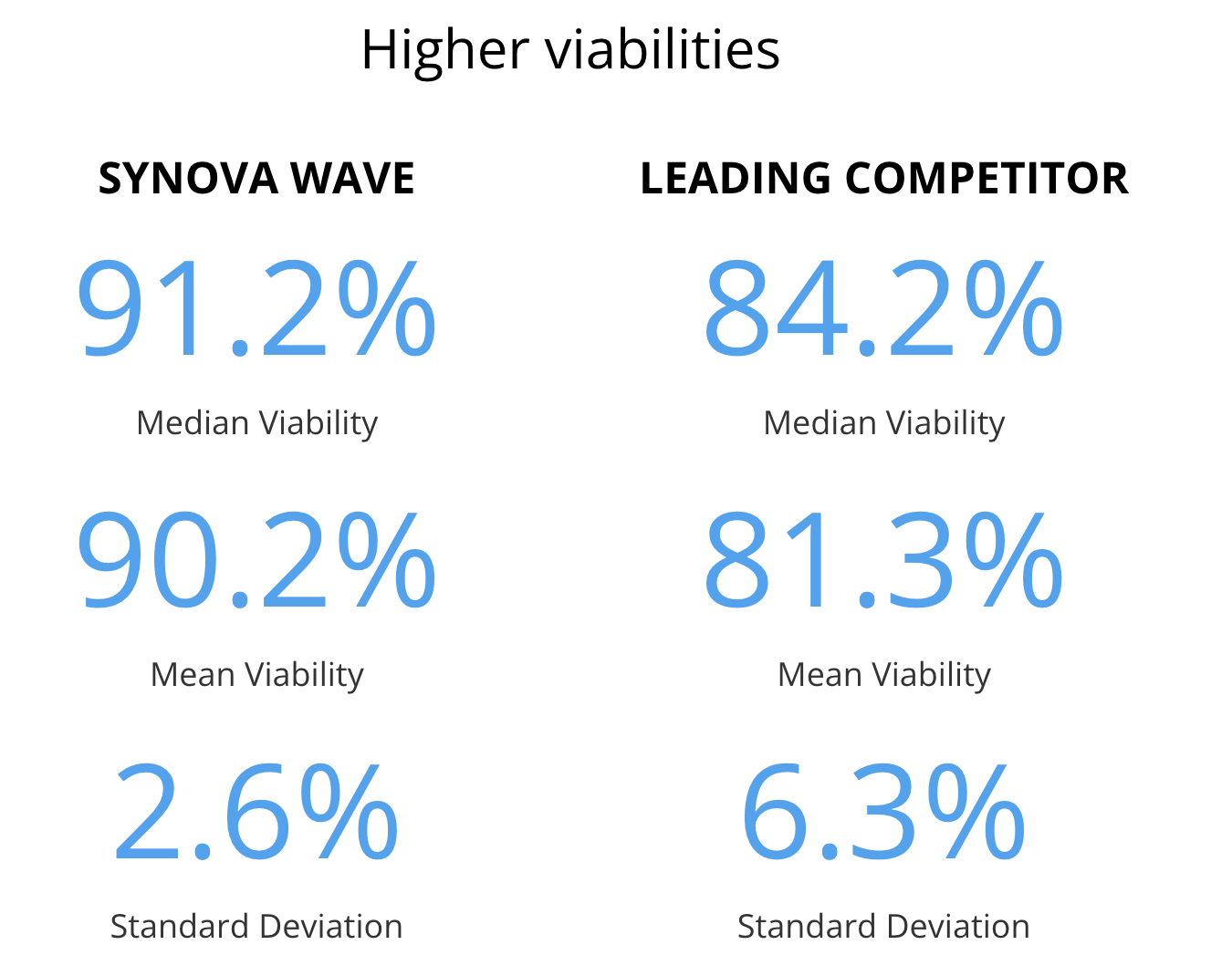
The system produces high-viability adipocytes and regenerative cells (e.g., ~90% viability), noticeably higher than many competitors.
-
Independent sources note the use of proprietary shockwave or “shock-assisted viable extraction” technology to mechanically separate stromal vascular fraction (SVF) and stem cells without enzymes, yielding substantial viable regenerative cells in minutes.
🧪 Non-Enzymatic & Minimally Manipulative
-
Unlike traditional enzymatic digestion (e.g., collagenase), the Synova process is non-enzymatic and minimally manipulative, which simplifies workflow and can help with regulatory compliance.
🧰 Automated & Sterile
-
The device incorporates an automated processing platform and disposable sterile cartridge, facilitating ease of use in the clinic and minimizing contamination risk.
💉 Intended Uses & Applications
According to Lionheart Health’s product listing, the adipose-derived stromal fraction and regenerative cells produced by the Synova WAVE can be used in treatments aimed at:
-
Joint health
-
Sexual health
-
Skin regeneration
-
Hair regeneration
-
Breast augmentation
(all via autologous cell use at the point of care)
🩺 Regulatory & Clinical Status
-
The Synova WAVE is described on Lionheart Health’s site as an FDA 510(k) cleared device (implying it has received regulatory clearance for specific clinical use).
-
Other sources note that Synova Life Sciences has pursued FDA regulatory pathways and ongoing development for broader clinical adoption.
🧠 Company Background — Synova Life Sciences
-
Synova Life Sciences is a biotech company based in California focused on developing devices that quickly and safely harvest stem cells from adipose tissue at the point of care.
-
The technology aims to make personalized regenerative medicine more accessible by enabling clinicians to extract a patient’s own cells quickly without complex lab processes — enabling same-day collection and therapeutic use.
🧠 In Summary
Bedside Adipose Tissue Processing Device
(collect stem cells, endothelial cells, regenerative proteins from fat tissue collected via a mini-lipo suction technique)
https://www.synovalife.com
The adipose tissue derived stromal fraction with stem cells, endothelial cells and proteins from this FDA 510K cleared device can be used for..
1. Joint health.
2. Sexual health,
3. Skin regeneration.
4. Hair regeneration.
5. Breast augmentation.
Adipocytes obtained through the Synova WAVE process stained with Calcein AM and DAPI showing healthy metabolic activity and high viability. Viability was 91.2% median, 90.2% mean, with a standard deviation of 2.6%
Adipocytes obtained through processing of the leading competitor stained with Calcein AM and DAPI showing much lower metabolic activity and significantly fewer viable adipocytes compared to the donor-matched sample obtained from the Synova WAVE. Viability from the leading competitor was 84.2% median, 81.3% mean with a standard deviation of 6.27%.





Reviews
There are no reviews yet.